News classification
Contact us
- Add: china-hena-hebi
- Tel: 13333923694(微信号)
- Fax: 13333923694
- Email: 908462358@qq.com
This article mainly introduces the principle and measurement method of calorific value from seven parts: the unit of heat and temperature, the basic principle of oxygen bomb calorimetry, the equipment requirements of calorimetry systems, the definition of high and low heat generation, the calibration of heat capacity, result calculation, and precision and accuracy evaluation.
A unit of heat and temperature
1 heat unit
The past and current units of heat in our country were calories and joules.
Joule (represented by J)
The work done by moving the point of action in the direction of the force by 1 Joule=1 Newton force.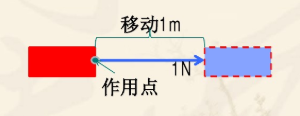
Card (represented by cal)
1 calorie is the amount of heat required to raise the temperature by 1 ℃ by 1 gram of pure water at standard pressure. Later, scientists discovered that the specific heat capacity of water varies at different temperatures, and the value of 1 calorie is not absolute.
The heat required to transfer 1g of water from 1 ℃ (from 14.5 ℃ to 15.5 ℃) at 1 atmospheric pressure is 1 calorie, which is approximately 4.1855 joules and is called 15 degree calories.
The heat required to transfer 1g of water from 1 ℃ (from 19.5 ℃ to 20.5 ℃) at 1 atmospheric pressure is 1 calorie, which is approximately 4.1816 joules, called 20 degree calories.
As can be seen from the above, 15 degree calories are not equal to 20 degree calories.
Therefore, from two definitions, it can be seen that the Joule is only related to the magnitude of the force, the direction of the force, and the distance of movement, and does not change with temperature. The calorie changes with temperature, and the numerical value is not absolute. Therefore, modern science no longer uses the calorie as a measure of energy and heat, but instead uses the international unit of Joule.
2 temperature units
The commonly used temperature units in our country are Celsius and Kelvin.
Celsius (expressed in ℃)
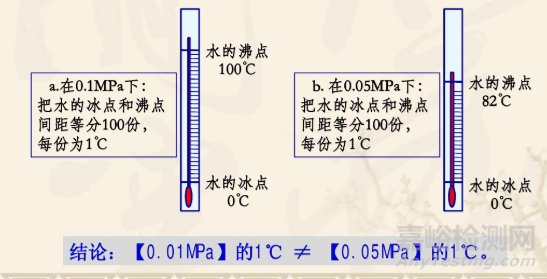
Celsius degree was established by the Swedish man Sersius in 1742. He set the melting point of water at standard atmospheric pressure to 100 degrees and the boiling point to 0 degrees, and divided the intervals between the mercury columns in the glass capillary into 100 grids, with each grid set at 1 ℃. Due to his unfamiliarity with usage, his student, the French man Chris, reversed the temperature values of the two fixed points, with a freezing point of 0 degrees and a boiling point of 100 degrees.

后来科学家发现水在不同大气压条件下的沸点不同,1℃的数值并不绝对。
Kelvin (represented by K)
The thermodynamic temperature is determined using an ideal gas: in order to make the scale unit of the Kelvin thermometer consistent with the scale unit on the Celsius thermometer, we divide the absolute temperature of the ideal gas and the triple point of water into 237.16 parts, each set at 1K (Calvin), so that the degree on the Kelvin thermometer is equal to the degree on the Celsius thermometer.
At standard atmospheric pressure, due to the temperature corresponding to the triple point of water being+0.01 ℃, the thermodynamic temperature corresponding to 0 ℃ is 273.15K, and the Celsius temperature corresponding to 0K is -273.15 ℃.
From the above, it can be seen that the temperature in Celsius is related to atmospheric pressure conditions. The value of 1 ℃ is not absolute, but thermodynamic temperature is independent of atmospheric pressure. Therefore, thermodynamic temperature is used as the temperature unit internationally. However, due to the fact that most people around the world live under standard atmospheric pressure conditions, Celsius temperature is still widely used.



